potassium electron configuration|Electron Configuration Chart of All Elements (Full Chart) : Tagatay Ene 21, 2021 — The basic elemental potassium is a silvery-white soft alkali metal that oxidizes instantly in the air and reacts vigorously with water. With water, it generates sufficient heat that ignites hydrogen which gets .
The Schools Division Office-Navotas through the Schools Governance Operations Division conducted an activity titled “Healthy and Positive Aging Seminar for Senior Citizens of SDO-Navotas” on June 16-18, 2022. via .
potassium electron configuration,Learn how to write the electron configuration for potassium using the period table or an electron configuration chart. See the step-by-step process and the video tutorial for more .
119 rows — Mar 23, 2023 — Find the electron configuration of potassium .Nob 18, 2013 — Learn how to write the electron configuration for Potassium (K) using the electron configuration chart. The video shows the step-by-step process and the final notation for K.Learn how to write the electron configuration of potassium, a chemical element with atomic number 19 and symbol K. Find out its properties, characteristics, isotopes and more.Hun 21, 2019 — In this video we will write the electron configuration for K+, the Potassium ion. We’ll also look at why Potassium forms a 1+ ion and how the electron config.Ene 21, 2021 — The basic elemental potassium is a silvery-white soft alkali metal that oxidizes instantly in the air and reacts vigorously with water. With water, it generates sufficient heat that ignites hydrogen which gets .

Thus, potassium has an electron configuration of [Ar]4s 1. Hence, potassium corresponds to Li and Na in its valence shell configuration. The next electron is added to complete the 4s subshell and calcium has an .
Electron configuration of Potassium is [Ar] 4s1. Possible oxidation states are +1. Potassium is one of the alkali metals. All of the alkali metals have a single valence .The electron configuration for potassium is 1s2 2s2 2p6 3s2 3p6 4s1. Knowing the electron configuration of potassium aids in predicting its chemical reactivity and bonding .A configuração eletrônica do potássio é [Ar] 4s1. A configuração do potássio é realizada de acordo com as regras derivadas de Klechkovskaya, a saber: Ar: 4s1, levando em consideração que é pela configuração dos elementos que é determinada a maneira como os elétrons são estruturados nos átomos do elemento .
Element Potassium (K), Group 1, Atomic Number 19, s-block, Mass 39.098. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. . Members of a group typically have similar properties and electron configurations in their outer shell. Period A horizontal row in the periodic table. The atomic number of each element .Mar 17, 2023 — The electron configuration of this titanium ion(Ti 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6. This electron configuration shows that the titanium ion(Ti 4+) has acquired the electron configuration of argon and it .

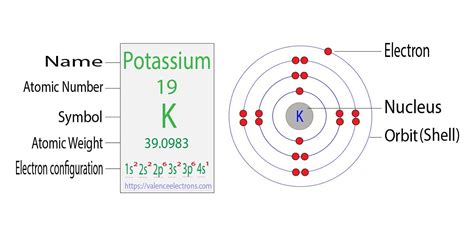
Hun 27, 2024 — This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation.. But wait .Electron Configuration Chart of All Elements (Full Chart)Hun 27, 2024 — This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation.. But wait .The full electron configuration of Potassium (K) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1. The abbreviated form - [Ar]4s 1 - means the electron configuration of Argon (Ar), plus one electron in the 4s orbital. Argon has 18 electrons. The one additional electron configuration completes the picture for 19 electrons of Potassium.The arrangement of electrons in various energy levels of an element is called electronic configuration. The maximum number of electrons which can be accommodated in any shell of an atom is given by 2n 2. Atomic number of potassium = 19; Electronic configuration = 2,8,8,1 (K- shell = 2 electrons, L-shell = 8 electrons, M - shell = 8 .
The first three quantum numbers of an electron are n=1, l=0, m l =0. Only two electrons can correspond to these, which would be either m s = -1/2 or m s = +1/2. As we already know from our studies of quantum numbers and electron orbitals, we can conclude that these four quantum numbers refer to the 1s subshell.Mar 26, 2023 — The arrangement of electrons in nobelium in specific rules in different orbits and orbitals is called the electron configuration of nobelium. The electron configuration of nobelium is 5f 14 7s 2, if the electron arrangement is through orbitals. Electron configuration can be done in two ways. Electron configuration through orbit (Bohr .Potassium electron configuration. ← Electronic configurations of elements . K (Potassium) is an element with position number 19 in the periodic table. Located in the IV period. Melting point: 63.7 ℃. Density: 0.86 g/cm 3. Electronic configuration of the Potassium atom: 1s 2 2s 2 .
Ene 21, 2021 — Potassium Electron Configuration: Potassium is a chemical element. Its symbol is K that is taken from Neo-Latin kalium. The atomic number of potassium is 19. Firstly it was isolated from potash .The full electron configuration of potassium is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1; Shorthand Electron Configurations. Using potassium as an example again: The nearest preceding noble gas to potassium is argon; This accounts for 18 electrons of the 19 electrons that potassium has; The shorthand electron configuration of potassium is [Ar] 4s 1Mar 18, 2023 — The valency of the element is determined by electron configuration in the excited state. Here, bromine has three unpaired electrons. So in this case, the valency of bromine is 3. Bromide ion(Br .potassium electron configurationNob 11, 2016 — Electron Configuration - Download as a PDF or view online for free. . potassium (K: Z = 19) (b) molybdenum (Mo: Z = 42) (c) lead (Pb: Z = 82) Use the atomic number for the number of electrons and the periodic table for the order of filling for electron orbitals. Condensed configurations consist of the preceding noble gas and outer .The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing atomic number Z. Electron configuration of Potassium is [Ar] 4s1. Possible oxidation states are +1.
May 24, 2024 — The electron configuration for potassium is 1s2 2s2 2p6 3s2 3p6 4s1. It has 19 electrons, with the last electron occupying the 4s orbital. Which element has the following ground state .potassium electron configuration Electron Configuration Chart of All Elements (Full Chart)Il existe 25 isotopes connus du potassium, dont trois se produisent naturellement : 39K (93,3 %), 40K (0,0117 %) et 41K (6,7 %). Le potassium 39 est composé de 19 protons, 20 neutrons et 19 électrons. Potassium - Protons - Neutrons - Électrons - .
カリウムの電子配置は [Ar] 4s1。 カリウムの配置は、Klechkovskayaから導出された規則、つまり、Ar:4s1に従って実行されます。
potassium electron configuration|Electron Configuration Chart of All Elements (Full Chart)
PH0 · What is the electron configuration of potassium? Chemistry Q&A
PH1 · Potassium – Protons – Neutrons – Electrons – Electron
PH2 · Potassium Electron Configuration (K) with Orbital
PH3 · Potassium Electron Configuration
PH4 · K+ Electron Configuration (Potassium Ion)
PH5 · How to Write the Electron Configuration for Potassium (K)
PH6 · How To Write Electron Configuration For Potassium
PH7 · Electron configuration of potassium
PH8 · Electron Configuration for Potassium (K, K+ ion)
PH9 · Electron Configuration Chart of All Elements (Full Chart)
PH10 · 3.1: Electron Configurations